Food Illness Caused by Ground Beef 911

Contribution of Foods and Poor Food-Treatment Practices to the Brunt of Foodborne Infectious Diseases in French republic
one
National Veterinary School of Alfort, 94700 Maisons-Alfort, France
ii
French Bureau for Food, Ecology and Occupational Wellness & Safe (Anses), 94700 Maisons-Alfort, France
3
Section of Psychology, Parisian Laboratory of Social Psychology (LAPPS), University Paris Nanterre, 92000 Nanterre, France
4
French National Public Health Agency, 94415 Saint-Maurice, French republic
5
Laboratory of Parasitology—Mycology, EA ESCAPE, University of Reims Champagne-Ardenne, 51100 Reims, France
*
Author to whom correspondence should be addressed.
†
In addition to the authors: Sandrine Blanchemanche, Laure Bonnaud, Michel Gautier, Françoise Gauchard, Lydiane Nabec, Louis-Georges Soler.
Received: 30 September 2020 / Revised: 3 November 2020 / Accustomed: 6 November 2020 / Published: 11 November 2020
Abstract
The foodborne disease burden (FBDB) related to 26 major biological hazards in France was attributed to foods and poor nutrient-handling practices at the final food preparation step, in lodge to develop constructive intervention strategies, specially nutrient safety campaigns. Campylobacter spp. and not-typhoidal Salmonella accounted for more than than 60% of the FBDB. Approximately 30% of the FBDB were attributed to 11 other hazards including leaner, viruses and parasites. Meats were estimated as the main contributing food category causing (50–69%) (CI90) of the FBDB with (33–44%), (9–21%), (iv–20%) (CI90) of the FBDB for poultry, pork and beef, respectively. Dairy products, eggs, raw produce and complex foods caused each approximately (five–20%) (CI90) of the FBDB. When foods are contaminated before the final training step, we estimated that inadequate cooking, cantankerous-contamination and inadequate storage contribute for (nineteen–49%), (vii–34%) and (9–23%) (CI90) of the FBDB, respectively; (15–33%) (CI90) of the FBDB were attributed to the initial contamination of prepare-to-eat foods—without any contribution from final nutrient handlers. The thorough implementation of proficient hygienic practices (GHPs) at the final food preparation step could potentially reduce the FBDB by (67–85%) (CI90) (mainly with the prevention of cantankerous-contagion and adequate cooking and storage).
1. Introduction
The burden of foodborne illnesses (i.e., estimates of the annual numbers of foodborne illnesses and associated hospitalizations and deaths) was estimated in France for 15 major pathogens [one]. These pathogens were estimated to business relationship for 1.v 1000000 cases of foodborne illnesses (90% credible interval, CI90, 1.three–2.ii million) each yr in France. Amid these foodborne pathogens, Campylobacter spp., non-typhoidal Salmonella and norovirus were responsible for 73% of all illnesses. These estimates are of master importance to prepare priorities for surveillance, prevention and command strategies [1].
Regarding the prevention and intervention options, information technology is also essential to aspect the disease burden to food bolt and nutrient-treatment practices. Assay of foodborne outbreaks investigations has been shown useful to aspect illness acquired past several pathogens [2,3,four,5]. Even so, even though the notification of foodborne outbreaks is mandatory in France, these notified cases constitute a small part of the total number including sporadic cases. For instance, 119 outbreaks of salmonellosis were notified in France in 2016 [6] accounting for 1047 cases of illnesses among the total estimated number of 183,000 cases (CrI90 102,000–388,000) corrected for underreporting and including sporadic cases. Moreover, the proportion of outbreaks for which the transmission route is known with stiff evidence is low, strong-bear witness outbreaks in the European Matrimony (Eu) accounted in 2018 for 709 xiii.eight% of all reported outbreaks [7]. As a result, the proportion of salmonellosis cases for which the manual route is known with strong evidence is very low. Source attribution studies using microbiological subtyping were performed in France for Salmonella [7] and Campylobacter spp. [8,9]. These studies are interesting with which to assess the relative contribution of different nutrient-fauna reservoirs and to justify control measures at the primary production just are unable to place implicated food vehicles and nutrient-treatment practices.
Some foodborne illnesses are associated with poor handling practices at the final grooming step (in household or food service establishments) such as improper storage, inadequate cooking, or cross-contagion [10]. The importance of the role of consumers is too confirmed by data from foodborne outbreak investigations in France. Nigh i-third of foodborne outbreaks reported in France occur inside the family (between 26% to 39% depending on the year) [six]. Thus, food rubber information aimed at consumers could help reduce the foodborne disease burden. Likewise, training programs are frequently mandatory for professional food handlers [eleven].
The major measures to command pathogens such every bit cleaning and disinfection of bounds, equipment and hands, or advisable storage and cooking temperature are well known [12,13] but the attribution of the foodborne illness burden (FBDB) to the poor food-handling practices needs to exist quantified in lodge to develop targeted prevention campaigns.
The objective of this written report was to place key foods and food-handling practices associated with each foodborne pathogen affliction in France in lodge to develop effective intervention strategies. This approach will attribute the FBDB amidst foods and nutrient-handling practices at the preparation footstep at home and in food service establishments.
2. Materials and Methods
2.one. Working Grouping
A working group was constituted by the French Bureau for Food, Environmental and Occupational Health and Safety (ANSES). Attention was paid to fugitive conflict of interests and to ensuring that the expertise of the 15 expert members adequately reflected the aims of the study: foodborne diseases (epidemiology, infectious diseases, risk assessment, food science, bacteriology, virology, parasitology) and social sciences (sociology, public policy, advice sciences, social psychology). The collective expertise was conducted on the basis of French epidemiological information to estimate the brunt of foodborne diseases and on a literature search on the risk factors (foods and practices) of foodborne diseases. The literature data used to complete the source attribution are presented in Appendix A.
2.2. Estimating the Burden of Foodborne Diseases
The FBDB in French republic was assessed by combining the incidence of major foodborne pathogens and the severity of the resultant diseases. The 26 major foodborne pathogens or hazards (including histamine, and differentiating the congenital and acquired forms of toxoplasmosis) considered in the study are presented in Table 1 and Table 2. An arroyo based on scoring was applied to characterize the incidence and the severity of diseases. The apply of broad categories for the scoring indirectly takes into account information uncertainties [fourteen] for incidence and severity.
The incidence of foodborne diseases was taken from a study performed in French republic for the flow 2008–2013 [1,15] for 15 pathogens among the 26 hazards or was estimated from surveillance information nerveless by the National Public Wellness Agency (Santé Publique France) and by National Reference Centers (NRCs). The estimated annual incidence of diseases per 100,000 persons in France was scored with a decimal logarithm scale every bit follows: score = 0, <0.01 cases; score = one, 0.01–0.ane cases; score = 2, 0.1–1 cases; score = 3, 1–10 cases, score = 4, 10–100 cases, and score =5, >100 cases.
The severity of diseases was expressed as the disability-adapted life year (DALY) per case which summarizes the impact of morbidity and mortality of diseases in a unmarried measure out [16]. The DALYs, expressing so the numbers of years lost due to disability or early on death, were too categorized using a decimal logarithm scale every bit follows: score = 1, <ten; score =2, 10–99; score = 3, 100–999; and score = 4, ≥thou per grand cases of illnesses. Published studies [17,xviii,nineteen] were primarily used in deriving the score of the DALYs for diseases. Expert opinion of epidemiologists and experts in infectious diseases was subsequently used when no estimate was published for some diseases or when experts estimated that published DALYs were not relevant. In these cases, the attributed scores were derived from those of diseases characterized by similar symptoms.
The disease burden was expressed past adding incidence and severity scores (which is equivalent to the multiplication of raw estimates for incidence and severity on a linear scale). The FBDB expresses then a public health metric equivalent to DALYs. The percentage of burden attributable to each hazard was then estimated as the fraction of the exponent with base ten of each adventure score among the total of exponents of scores.
2.iii. Attribution of Foodborne Disease Burden to Foods and Practices
For each foodborne pathogen, the main foods considered as significant exposure routes were identified based on literature data and past expert opinion from the working group (Appendix A). Food mishandling practices that could lead to foodborne diseases were also identified for each hazard–nutrient combination. These practices contribute to the manifestation of foodborne diseases when handling initially contaminated foods. The contributing factors were classified equally (i) cantankerous contagion, (ii) inadequate washing and disinfection of produce, (iii) inadequate processing during domestic preparation (acidification, h2o activeness, fermentation) or cooling, (iv) inadequate freezing (insufficient temperature and/or freezing duration to destroy parasites potentially present in foods), (v) inadequate cooking (including reheating), (vi) inadequate storage (temperature and/or shelf-life).
To estimate the relative contribution of each food and nutrient categories to the disease burden in France, the brunt of each hazard was attributed to related foods past taking into account the uncertainty in their significance for the transmission of the hazard (i.e., the burden is divided by the number of foods implicated). This approach was used considering no quantitative data are bachelor to justify a more accurate source attribution process for numerous foodborne hazards or, when available, quantitative estimates are characterized by a swell uncertainty. For case, salmonellosis outbreaks in French republic are linked to eggs and raw egg products in approximately 50% of cases, pork meats are implicated in 15% of cases, raw milk cheeses are implicated in 10% of cases, poultry meats are implicated in less than 10% and beef meats are responsible for 5% of outbreaks [xx]. This repartition can be thoroughly modified when looking at sporadic cases instead of outbreaks. David et al. [seven] estimated that sporadic cases occurring in French republic in 2005 were also related to eggs in 50% of cases, but pork meats deemed for approximately 25% of cases, as are poultry meats, which is higher than estimates obtained with outbreaks. On the opposite, the cattle reservoir was implicated in less than 1% of desultory cases. In another written report, attributing sporadic salmonellosis to sources, the primary part of pigs and poultry was confirmed but the layers were estimated to business relationship for but 7% of cases in France [21]. We then estimated dubiety intervals for the relative contribution of the main identified food past performing Monte-Carlo simulations (10,000 runs) with Microsoft Excel (Microsoft Corporation, Redmond, DC, Usa). Uniform distributions were used for the relative contributions of each food on the burden with minimum contributions of 5% and maximum contributions equal to (100 − (n − 1) × v)%, where north is the number of implicated foods. These simulations allowed the interpretation of ninety% uncertainty intervals (5th and 95th percentiles, CI90) for the contribution for each food.
Due to the lack of quantitative data, the relative affect of poor nutrient-handling practices on the disease brunt was also assumed to be the same, i.e., uncertain range for the impact of contributing practices, for each chance–food combination. Monte-Carlo simulations (10,000 runs) with Microsoft Excel (Microsoft Corporation) were performed using compatible distributions with a minimum relative contribution of five% for each food-treatment mistake to approximate 90% uncertainty intervals of the impact of contributing practices.
3. Results
iii.1. Estimation of the Foodborne Disease Burden in France
The incidence and severity estimates of foodborne disease are shown in Tabular array 1 and Tabular array ii respectively. The start grouping of hazards (Campylobacter spp. and Salmonella) accounts for 64% of the FBDB. The second group, including 11 pathogens: toxin-producing bacteria (Bacillus cereus, Clostridium perfringens, Staphylococcus aureus), Shiga toxin-producing Escherichia coli (STEC), Listeria monocytogenes, Yersinia enterocolitica, foodborne viruses (norovirus, hepatitis A and Eastward viruses), and Toxoplasma gondii (built and acquired infections), accounts for a 35% of the FBDB. The remaining 13 hazards, including 8 parasites, account for i% of the FBDB.
3.2. Attribution of Disease Burden to Foods and Practices
The major exposure routes and nutrient-handling practices were identified for each foodborne hazard (Table three). Appendix A gives the details of the data used by the working grouping to justify the expert opinion about source attribution for each foodborne hazard. These are the foods most frequently contaminated or responsible for the majority of outbreaks.
Figure 1 illustrates the burden distribution past food category and consumer practices. Meats are estimated as the main contributing food category causing (l–69%) (CI90) of the FBDB (Table 4). Amidst them, poultry meat is the primary correspondent ((33–44%) (CI90) of the FBDB). Dairy products, eggs, raw produce and complex foods are estimated to cause each approximately (5–xx%) (CI90) of the FBDB. Seafood are estimated as minor category with (ane–vi%) (CI90) of the FBDB. Raw foods (eastward.chiliad., footing meat, raw milk, raw eggs products, raw fish and shellfish, etc.) business relationship for (23–41%) (CI90) of the FBDB.
Inadequate cooking and cantankerous contamination account for (19–49%) (CI90) and (seven–34%) (CI90) of the FBDB, respectively (Tabular array 5). These poor treatment practices are mainly related to meats (Effigy 1). Inadequate storage of various foods (Effigy 1) is responsible for (ix–23%) (CI90) of the FBDB. Inadequate washing and disinfection of produce accounts for (2–13%) (CI90) of the FBDB, and error in food processing and inadequate freezing take small-scale impacts. Some foodborne illness cases are linked to the consumption of set-to-eat foods that are initially contaminated without whatsoever contributing practice of food handlers at the preparation footstep. These situations account for (xv–33%) (CI90) of the illness burden. This is for instance the case of raw shellfish contaminated past norovirus or raw ground beef contaminated by STEC or Salmonella.
The thorough implementation of good hygienic practices (GHPs) at the final preparation step could reduce by (67–85%) (CI90) the FBDB (Table v) essentially by avoiding cantankerous-contamination, and with a correct storage and cooking which account for (lx–78%) (CI90) of the burden.
iv. Discussion
The foodborne disease burden (FBDB) related to 26 biological hazards in France was estimated and attributed to foods and poor nutrient-treatment practices at the terminal food training footstep. We estimated that approximately 60% of the FBDB respective in this study to DALYs was attributed to Campylobacter spp. and non-typhoidal Salmonella. The importance of these two pathogens was also observed by Hoffman et al. [28] who estimated that approximately 50% of the quality-adapted life year (QALY) loss in the United states of america was caused by these two bacteria. Kirk et al. [18] also estimated that salmonellosis and campylobacteriosis account for approximately fifty% of the DALYs for the European region. The incidence estimates used in this study are mainly derived from a French written report conducted by the National Public Health Agency [1]. For most pathogens the estimated number of cases per 100,000 persons are similar to other estimates obtained in the Netherlands and United states [17,29]. Nevertheless, differences can be noticed. For instance, the incidence of cryptosporidiosis, cyclosporiasis and giardiasis, that were estimated from surveillance data from National Reference Centers and laboratory surveillance networks (Table 1), are specially low in France. The incidences are approximately 100 lower than those published for other developed countries [18,29,30]. French estimates were based on notified cases and the bodily incidence for these protozoan infections was probably underestimated but no French study was available to estimate underdiagnosis and underreporting factors. It is worth noting that, because scores based on a log10 scale ranging from ane to 5 were used, even though some uncertainty is associated with incidence estimates, the scores would by and large remain the same whatever the country under consideration.
The estimated DALYs published by Havelaar et al. [17] for the Dutch population in 2009 and by the Foodborne Disease Burden Epidemiology Reference Group (FERG) established by the World Health Arrangement (WHO) for the Europe A zone [18,nineteen] were used every bit no French study estimating the severity of foodborne diseases was bachelor. Nevertheless, the severity of toxoplasmosis published past Havelaar et al. [17] and estimated at 3170 DALYs per 1000 cases was considered as over-estimated past the experts of the working group given the very low rate of severe forms of congenital toxoplasmosis in France [31]. A score of ii (between ten and 99 DALYs per 1000 cases) was proposed in accord with the estimate of Kirk et al. [18] of 60 DALYs per 1000 cases (Table two). Based on observed clinical manifestations in France, the severity of fascioliasis provided by Torgerson et al. [nineteen] estimated at 9000 DALYs per 1000 cases was also considered every bit overestimated and the working group proposed rather a score of two (Table ii).
In our study, meats are estimated as the main contributing food category which is in accordance with the estimates of Batz et al. [32] where meats were responsible for nearly lx% of the QALY loss in the Usa. More specifically, poultry, pork and beef were responsible for 24%, 13% and 10% of the QALY loss, respectively [32]. In France, comparable estimates of (34–44%) (CI90), (9–21%) (CI90) and (4–20%) (CI90) of the foodborne disease burden were attributed to poultry, pork and beefiness meats, respectively. The importance of dairy products is similar in France ((5–22%) (CI90) of the burden) and in the United states of america (9% of the QALY loss, [32]). Raw produce and complex foods, with (six–20%) (CI90) and (eight–12%) (CI90) of the burden in France, respectively, are also similar to the United states where they deemed for 10% and 12% of the QALY loss, respectively [32]. Seafood and eggs were regarded as minor sources in both countries, representing less than 10% of the disease burden or the QALY loss [32]. Finally, nosotros can too emphasize the importance of raw foods in France that accounted for (23–41%) (CI90) of our gauge of the disease brunt.
Good opinion was used to place the primary exposure routes leading to foodborne disease because "difficult" data were not available. The estimates are then sensitive to the subjective judgment of experts and results from other food source attribution studies are not fully comparable because of differences in geographic coverage, methods and food categorization [33]. For case, our study kept poultry meat every bit the sole main source of campylobacteriosis (Tabular array three) while Hoffmann et al. [33] identified besides beef, dairy, and pork as sources in the EUR A subregion. However, these sources were less significant and were estimated to business relationship for 15% to xx% of foodborne campylobacteriosis. For salmonellosis, the illnesses in the EUR A subregion are mainly attributed to eggs, pork and poultry meats past Hoffmann et al. [33] while our report allocated uniformly the salmonella FBDB to a greater list of nine food products (Table 3). However, doubtfulness bounds were constructed around the estimates to take into account the dubiousness near the relative importance of each nutrient source. The attribution of foodborne disease to foods was so estimated using uniform distributions for the relative importance of each nutrient considered as relevant for the unlike hazards and contributing to at least five% of the burden related to each take a chance. Although the incertitude intervals can be large for a specific pathogen–food combination, the doubtfulness intervals for nutrient categories derived from these distributions are relatively narrow (Table 4). For example, the burden estimate for cooked and raw ground beefiness is (2–13%) (CI90) while the estimate for beefiness is (iv–20%) (CI90), and for the whole meat category is (50–69%) (CI90) (Table 4). These results show that the relative contribution of large food categories is not much affected by the uncertainty about the specific food sources even if the doubtfulness bounds of these are broader. Although the doubt premises are wider for food-handling practices, the phenomenon is quite similar (Table v). Inadequate cooking, which is the primary contributing factor accounts for (xix–49%) (CI90) of the FBDB.
Quantitative microbial take chances assessment studies often addressed the impact of some particular consumer practices on reducing foodborne risk e.g., issue of hygiene practices of consumers during the preparation of chicken meals on the salmonellosis and campylobacteriosis risks [34,35] or effect of domestic refrigerator temperature on the listeriosis risk [36] or exposure to high levels of B. cereus [37]. Other studies quantified the effect of specific hygiene practices on the microbial load of handled foods, e.g., consequence of hygiene measures applied to cutting board, cutlery, and hands on the microbial transfer from meat to salad [38]. To our knowledge, our report is the starting time because the overall attribution of the poor handling practices to FBDB. According to our study, cross-contamination, inadequate cooking and storage are responsible for (lx–78%) (CI90) of the FBDB. These practices were besides identified by previous studies (e.g., Medeiros et al. [12] and Taché and Carpentier [13]) but their relative contributions to the FBDB were not assessed.
Food safe prevention appears to be a domain where behavioral change could effectively reduce the FBDB. The potential reduction in affliction burden when correctly applying adept hygienic practices (GHPs) at the final training step is, withal, hard to estimate. It depends on the efficiency of command measures to limit the transfer of microorganisms, to inactivate them or to inhibit their multiplication as well as on the inclination of nutrient handlers to use GHPs correctly. We can assume that the prevention of cross-contamination and adequate washing of produce with water containing a disinfectant would be partially effective in reducing the FBDB. Indeed, previous studies have reported that both these interventions, i.east., prevention of cross-contamination [39,40] and washing with a disinfectant solution [40,41] can but partially reduce microbiological load. On the other hand, the correct implementation of freezing, cooking, adequate storage and processing or acceptable cooling could accept a college touch on to reduce FBDB. For example, Pouillot et al. [42] showed that decreasing the shelf-life of cold smoked salmon or maintaining the average refrigerator temperature at 4 °C reduced the number of listeriosis cases by more than 75%. Regarding cooking, Smith et al. [43] showed that cooking ground beefiness to an internal temperature of at least 71 °C decreases the boilerplate probability of East. coli O157:H7 infection in Canada past a factor of 105.
Beyond food condom crises, public wellness interventions aim to modify the behavior of food handlers. Educational food safe interventions focus on small-scale school or community samples [44]. Among all intervention techniques, health information campaigns or mass media campaigns are able to target the consumers' general population. The impact of health campaigns on population wellness has been estimated in other aspects of public health and condom (nutrition, cancer screening, road condom, and blood donation). According to published meta-analysis [45,46], the average health campaign (excluding campaigns that include legal coercion) changes the behavior of the target population according to a small-scale consequence in the short-term (r = five%) which varied by the target beliefs and context. To increase the effectiveness of the communication, it has been suggested that a advice strategy should combine different means of disseminating information (media, including social media, medical staff, consumer associations, etc.), address both the individual and their environment, and refer to a theoretically and empirically sound behavioral model [47].
5. Conclusions
This study aimed at estimating the office of specific foods every bit pathways contributing to foodborne disease burden and associated nutrient-handling practices for 26 pathogen hazards. Since no quantitative information were bachelor to perform the attribution process, good opinion was used leading to potential under- or overestimation of the implication of a particular food or practise. Nevertheless, the principal contributing food categories and practices could exist identified with reasonable confidence. These estimates constitute valuable information to considerately develop intervention strategies in guild to reduce the foodborne disease burden. To be most effective in reducing this FBDB, it is important to promote food safety messages to food handlers addressing the practices with the biggest impact in relation to FBDB i.e., cross-contagion, inadequate cooking and storage. These estimates are also useful to compare the potential impact of educational programs with interventions focusing on the food production chain, which could be a more effective take chances reduction strategy.
Author Contributions
Formal analysis, J.-C.A.; Investigation, J.-C.A., P.K., T.B., L.G., T.M., N.J.-D.Due south., I.V. and O.C.; Methodology, J.-C.A., P.1000., L.M. and Thousand.S.; Project assistants, P.Yard. and T.B.; Supervision, M.S. and O.C.; Visualization, L.G.; Writing—original draft, J.-C.A. and P.Thou.; Writing—review and editing, T.B., L.G., T.K., N.J.-D.S., I.5., M.S. and O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to give thanks ANSES staff and the members of the ANSES Working Group on Consumer information on foodborne biological risks (In add-on to the authors: Sandrine Blanchemanche, Laure Bonnaud, Michel Gautier, Françoise Gauchard, Lydiane Nabec, Louis-Georges Soler).
Conflicts of Involvement
The authors declare no conflict of involvement.
Appendix A. Source Attribution (Foods and Practices) for Foodborne Hazards in France
Bacillus cereus: Bacillus cereus outbreaks are mainly associated with the consumption of refrigerated and processed foods of extended immovability (REPFED) or composite meals containing grains. These estimations are in accord with previous estimates [48,49,fifty]. Outbreaks generally occur with inadequate storage conditions (cold chain disruption or exceeded shelf-life) or when grooming conditions are not well controlled (cooling and cooking) for home-made meals [51].
Brucella spp.: In enzootic areas, the foodborne transmission of Brucella spp. is linked to the consumption of contaminated unpasteurized milk or fresh not-ripened cheeses made with raw milk. Bacterial infection occurs when milk is inadequately heated earlier consumption merely no GHPs exist when consuming raw milk or raw milk cheeses. The just intervention available at the preparation footstep is to avoid the consumption of this kind of product [52].
Campylobacter spp.: The primary considered source of foodborne campylobacteriosis was poultry meat. International source attribution studies likewise associate Campylobacter infections with poultry [53,54,55]. Although many foods can serve as vehicle for Campylobacter since cantankerous contamination can occur during the food preparation, poultry meat was only considered as the ultimate source of contamination. Factors favoring the occurrence of campylobacteriosis are cross contamination but likewise inadequate cooking of poultry meat.
Clostridium botulinum : Botulism is mostly associated with the consumption of home-made pork meats and canned foods inadequately prepared, stored or cooked. Vacuum-packed REPFED inadequately stored and cooked were also associated with several cases of botulism [56,57]. A few cases of babe botulism were attributed to honey consumption [58].
Clostridium perfringens : Outbreaks involving C. perfringens are generally attributed to home-fabricated meals, specially beef meat based products [32,48,49,50]. These outbreaks require an error during the preparation (inadequate cooling or reheating) or the storage (inadequate temperature) steps.
Shiga toxin-producing E. coli (STEC): Foods associated with STEC infections are basis beef, raw milk and soft cheeses made with raw milk, and produce eaten raw. The importance of beef and produce is consistent with previous estimations [32,49,59]. Ground beef is incriminated when it is eaten raw (tartare) or insufficiently cooked. The only interventions at the preparation step for dairy products is to heat raw milk or to avoid eating raw milk products, especially for young children who demonstrate a higher host susceptibility. The contamination of produce tin exist reduced by applying washing and disinfection procedures.
Listeria monocytogenes : Listeriosis cases are related to the consumption of a large variety of foods. Near oft implicated foods are fix-to-swallow foods supporting the growth of the pathogen during the shelf-life. The major group of foods considered were cooked pork meats, soft cheese made with raw or pasteurized milk, cold smoked fish, crustaceans, raw vegetables and composite foods. Dairy products and deli meats were also regarded as major vehicles by Batz, Hoffmann [32]. Produce and seafood were considered as secondary sources [32]. Disruption of the common cold chain or exceeded shelf-life during storage are, then, contributing factors only listeriosis tin can also occur without food handlers existence at mistake when consumers exhibit a high host susceptibility.
Salmonella enterica, non-typhoidal: Salmonellosis can result from the consumption of numerous foods. The master considered foods were basis beef, chicken and pork meats, raw milk cheeses, raw eggs and egg products, raw produce. These implicated foods are consequent with published estimations ([32,48,49,59]. The favoring nutrient-treatment conditions (nutrient eaten raw or insufficiently cooked) and intervention strategies (cooking, washing and disinfection, removal) are the same as with STEC for meat products, cheeses and produce. By contrast with STEC, inadequate storage was also considered every bit a significant favorable factor for salmonellosis associated with meats. Infections implicating eggs are related to insufficient cooking and those linked to raw egg products are sometimes associated with cantankerous contagion and inadequate storage temperature.
Shigella : Foodborne shigellosis is mainly associated with meals prepared and mishandled in nutrient-service establishments [60]. The contamination tin exist due to cantankerous contamination with the food handlers during the preparation or prior to food grooming and no intervention strategy is and then available at the preparation step.
Staphylococcus aureus : South. aureus toxin is mainly associated with raw milk soft cheeses and numerous composite dishes [48,49,50]. No interventions strategies are available for food handlers when cheeses are contaminated. On the other hand, outbreaks linked to composite dishes are by and large related to inadequate temperature control during the grooming or the storage.
Vibrio parahaemolyticus : Foodborne infections involving V. parahaemolyticus are linked to shellfish eaten raw or insufficiently cooked. For raw bivalve mollusks, an inadequate storage temperature increases the probability of foodborne infection [61].
Yersinia enterocolitica : In France, Y. enterocolitica infections are mainly associated with pork meat [62]. Acceptable storage weather and a sufficient cooking are regarded every bit effective GHPs applicable by food handlers.
Histamine: Histamine is mainly produced in fish species with a high amount of histidine, peculiarly tuna from the Scombridae family. No intervention is available for food handlers at the grooming step since histamine is produced immediately afterward fishing.
Norovirus, hepatitis A virus: Foodborne transmission of norovirus and hepatitis A virus involves bivalve mollusks, raw produce (frozen or not) and extensively manipulated meals like sandwiches. These foods are the primary categories implicated in outbreaks [49]. Hoffmann et al. [59] and Batz et al. [32] identified produce and seafood as major foods for the manual of norovirus in the U.s.a.. No intervention strategies are available for food handlers when these foods are eaten raw. Adequately cooking bivalve mollusks is effective to destroy the viral particles. As for manipulated complex foods, the contagion generally occurs during food handling by cross contamination.
Hepatitis E virus: Foodborne manual of hepatitis E virus is linked to the consumption of hog liver products or food containing boar offal insufficiently cooked [63]. Seafood, fruit and vegetables were not considered as meaning routes [48].
Anisakis spp.: Anisakis spp. are parasites plant in infected fishes. A rapid evisceration of fishes is considered a skillful preparation exercise and is recommended to avoid the transit of larvae from abdominal cavity to muscles. Parasites tin can exist destroyed by adequate freezing or cooking which constitute relevant intervention strategies at the preparation stride [64].
Cryptosporidium spp., Cyclospora cayetanensis, Giardia spp.: These protozoan parasites are mainly associated with the consumption of raw produce. Reverse to Havelaar et al. [48], meats and shellfishes were considered as minor sources for Cryptosporidium and Giardia. Simply this was consistent with the estimations of Hoffman et al. [59] and Batz et al. [32] who also identified produce as the major source of contamination for Cryptosporidium and Cyclospora. Except for Cyclospora which is particularly resistant [65], the acceptable washing and disinfection of produce are useful intervention strategies applicable at the food-treatment pace to reduce the probability of infection.
Echinococcus multilocularis : The ingestion of Due east. multilocularis eggs is associated with the consumption of contaminated cherry fruits and berries or vegetables harvested near the ground. Eggs of the parasite are not eliminated at the food-handling step by washing and disinfection, and they are non completely inactivated by freezing [66]. Adequate cooking can destroy eggs of this parasite.
Fasciola hepatica : Fasciolosis is contracted by eating wild raw produce like watercress and dandelion. Parasitic metacercariae encysted on plants are highly resistant to washing and disinfection [67] then no intervention strategies are usable past final food handlers.
Taenia saginata : T. saginata is a parasite exclusively transmitted by beefiness meat. Adequate freezing or cooking are constructive GHPs to destroy Cysticercus bovis localized in meat [68].
Toxoplasma gondii : Toxoplasma oocysts are transmitted when eating contaminated raw vegetables. Wahsing and disinfection of vegetables is not effective to reduce the oocysts load present on produce [69]. Cysts are nowadays in tissue of infected animals. Eating lamb meat, meat from open-air pigs or horse meat is generally recognized as the source of toxoplasmosis when insufficiently cooked. Pork, beef and game meats were estimated as major routes in the USA [32,59]. Cysts can exist destroyed by freezing or adequate cooking of meats.
Trichinella spp.: Man trichinellosis is associated with the consumption of infected meats from open-air pigs, boars or game meats. Intervention strategies at the nutrient-handling step consisting in freezing or cooking can finer destroy the encysted larvae [lxx].
References
- Van Cauteren, D.; Le Strat, Y.; Sommen, C.; Bruyand, M.; Tourdjman, 1000.; Da Silva, N.J.; Couturier, E.; Fournet, N.; de Valk, H.; Desenclos, J.-C. Estimated Almanac Numbers of Foodborne Pathogen–Associated Illnesses, Hospitalizations, and Deaths, France, 2008–2013. Emerg. Infect. Dis. 2017, 23, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.G.; Vigre, H.; Mäkelä, P.; Hald, T. Using Outbreak Data for Source Attribution of Man Salmonellosis and Campylobacteriosis in Europe. Foodborne Pathog. Dis. 2010, 7, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.A.; Hoekstra, R.K.; Ayers, T.; Tauxe, R.Five.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities past using Outbreak Data, U.s.a., 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- IFSAC. Foodborne Illness Source Attribution Estimates for 2017 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter Using Multi-Year Outbreak Surveillance Data, United states of america. Bachelor online: https://world wide web.cdc.gov/foodsafety/ifsac/pdf/P19-2017-report-TriAgency-508.pdf (accessed on 28 August 2020).
- Pires, S.G.; Majowicz, S.; Gill, A.; Devleesschauwer, B. Global and regional source attribution of Shiga toxin-producing Escherichia coli infections using analysis of outbreak surveillance information. Epidemiol. Infect. 2019, 147, e236. [Google Scholar] [CrossRef]
- Santé Publique France. Données Relatives Aux Toxi-Infections Alimentaires Collectives Déclarées en French republic en 2016. 2018. Available online: https://www.santepubliquefrance.fr/media/files/01-maladies-et-traumatismes/maladies-infectieuses-d-origine-alimentaire/toxi-infections-alimentaires-collectives/tiac_donnees_2016 (accessed on three November 2020).
- David, J.; Sanders, P.; Bemrah, N.; Granier, S.; Denis, Thousand.; Weill, F.-X.; Guillemot, D.; Watier, L. Attribution of the French human Salmonellosis cases to the principal nutrient-sources according to the type of surveillance data. Prev. Veter Med. 2013, 110, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Thépault, A.; Méric, Thousand.; Rivoal, K.; Pascoe, B.; Mageiros, L.; Touzain, F.; Rose, V.; Béven, 5.; Chemaly, M.; Sheppard, S.K. Genome-Wide Identification of Host-Segregating Epidemiological Markers for Source Attribution in Campylobacter jejuni. Appl. Environ. Microbiol. 2017, 83, e03085-16. [Google Scholar] [CrossRef]
- Thépault, A.; Rose, V.; Quesne, S.; Poëzevara, T.; Beven, V.; Hirchaud, Eastward.; Touzain, F.; Lucas, P.; Méric, K.; Mageiros, L.; et al. Ruminant and craven: Important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018, eight, 9305. [Google Scholar] [CrossRef]
- World Health Organization. Five Keys to Safer Food Manual, Published by the WHO Department of Food Safety, Zoonoses and Foodborne Diseases. 2006. Available online: http://world wide web.who.int/entity/foodsafety/publications/consumer/manual_keys.pdf (accessed on 28 August 2020).
- Egan, M.; Raats, M.; Grubb, S.; Eves, A.; Lumbers, M.; Dean, M.; Adams, M. A review of nutrient safety and nutrient hygiene preparation studies in the commercial sector. Food Control. 2007, 18, 1180–1190. [Google Scholar] [CrossRef]
- Medeiros, L.C.; Kendall, P.; Hillers, Five.; Chen, M.; Dimascola, Southward. Identification and Classification of Consumer Food-Treatment Behaviors for Food Safety Education. J. Am. Diet. Assoc. 2001, 101, 1326–1339. [Google Scholar] [CrossRef]
- Taché, J.; Carpentier, B. Hygiene in the home kitchen: Changes in behaviour and bear upon of cardinal microbiological hazard control measures. Food Control. 2014, 35, 392–400. [Google Scholar] [CrossRef]
- Felício, M.D.Due south.; Hald, T.; Liebana, E.; Allende, A.; Hugas, M.; Nguyen-The, C.; Johannessen, One thousand.S.; Niskanen, T.; Uyttendaele, Grand.; McLauchlin, J. Risk ranking of pathogens in ready-to-eat unprocessed foods of not-animal origin (FoNAO) in the Eu: Initial evaluation using outbreak information (2007–2011). Int. J. Food Microbiol. 2015, 195, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Van Cauteren, D.; Le Strat, Y.; Sommen, C.; Bruyand, M.; Tourdjman, M.; Jourdan-Da, Due south.Due north.; Couturier, East.; Fournet, Northward.; De Valk, H.; Desenclos, J.C. Estimation de la morbidité et de la mortalité liées aux infections d'origine alimentaire en France métropolitaine, 2008–2013. Bull. Epidémiol. Hebdo. 2018, 1, two–10. [Google Scholar]
- Devleesschauwer, B.; Haagsma, J.A.; Angulo, F.J.; Bellinger, D.C.; Cole, D.; Döpfer, D.; Fazil, A.; Fèvre, Eastward.M.; Gibb, H.J.; Hald, T.; et al. Methodological Framework for World Health Organization Estimates of the Global Burden of Foodborne Disease. PLoS ONE 2015, 10, e0142498. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Haagsma, J.A.; Mangen, M.-J.J.; Kemmeren, J.Grand.; Verhoef, 50.P.; Vijgen, S.K.; Wilson, M.; Friesema, I.H.; Kortbeek, 50.Grand.; Van Duynhoven, Y.T.; et al. Disease brunt of foodborne pathogens in the Netherlands, 2009. Int. J. Food Microbiol. 2012, 156, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kirk, 1000.D.; Pires, S.M.; Blackness, R.Due east.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.50.; Hald, T.; et al. World Wellness Organization Estimates of the Global and Regional Affliction Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, Due north.; Speybroeck, North.; Willingham, A.Fifty.; Kasuga, F.; Rokni, Grand.B.; Zhou, 10.-N.; Fèvre, East.Chiliad.; Sripa, B.; et al. World Wellness Organisation Estimates of the Global and Regional Illness Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- ANSES. Stance of 16 November 2018 of the French Agency for Food, Environmental and Occupational Health & Safe Relating to the Source Attribution of Foodborne Diseases. Part 2, Assay of Epidemiological Information 2018. Available online: https://www.anses.fr/fr/system/files/BIORISK2015SA0162Ra-2.pdf (accessed on 28 Baronial 2020).
- Pires, South.M.; de Knegt, L.; Hald, T. Scientific/Technical Study Submitted to EFSA—Estimation of Relative Contribution of Different Nutrient and Brute Sources to Homo Salmonella Infection in the Eu. EFSA Support. Publ. 2011, 8, 184E. [Google Scholar]
- Mailles, A.; Garin-Bastuji, B.; Lavigne, J.-P.; Jay, M.; Sotto, A.; Maurin, Thou.; Pelloux, I.; O'Callaghan, D.; Mick, V.; Vaillant, V.; et al. Human brucellosis in France in the 21st century: Results from national surveillance 2004–2013. Médecine Et Mal. Infect. 2016, 46, 411–418. [Google Scholar] [CrossRef]
- Santé Publique France. Surveillance des Toxi-Infections Alimentaires Collectives—Données de la Déclaration Obligatoire. Available online: https://world wide web.santepubliquefrance.fr/maladies-et-traumatismes/maladies-infectieuses-d-origine-alimentaire/toxi-infections-alimentaires-collectives/donnees/#tabs (accessed on 3 November 2020).
- Centre National de Référence des Vibrions et du Choléra. Rapport Annuel d'activité 2014. 2015. Available online: https://www.pasteur.fr/fr/file/3296/download?token=CVo45czr (accessed on three November 2020).
- Eye de National de Référence des Virus des Hépatites à Transmission entérique. Rapport Annuel d'activité 2018. 2019. Available online: http://www.cnrvha-vhe.org/wp-content/uploads/2020/01/Rapport-activité-VHE-VHA-2018.pdf (accessed on 3 November 2020).
- Réseau CRYPTO-ANOFEL. Rapport Annuel d'activité 2014. 2015. Bachelor online: http://cnrcryptosporidioses.chu-rouen.fr/wp-content/uploads/sites/54/2018/06/2015_Rapport-annuel-2014-.doc (accessed on 2 November 2020).
- Van Cauteren, D. Estimation de la morbidité des infections d'origine alimentaire en French republic. Ph.D. Thesis, Université Paris Saclay, Saint-Aubain, France, 2016; p. 151. [Google Scholar]
- Hoffmann, S.; Batz, M.B.; Morris, J.G. Annual Toll of Affliction and Quality-Adjusted Life Twelvemonth Losses in the United States Due to 14 Foodborne Pathogens. J. Nutrient Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef]
- Scallan, Eastward.; Hoekstra, R.Chiliad.; Angulo, F.J.; Tauxe, R.V.; Widdowson, One thousand.A.; Roy, South.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011, 17, seven. [Google Scholar] [CrossRef]
- Thomas, M.One thousand.; Murray, R.; Flockhart, L.; Pintar, Thousand.; Pollari, F.; Fazil, A.; Nesbitt, A.; Marshall, B. Estimates of the Burden of Foodborne Affliction in Canada for thirty Specified Pathogens and Unspecified Agents, Circa 2006. Foodborne Pathog. Dis. 2013, 10, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Villena, I.; Ancelle, T.; Delmas, C.; Garcia, P.; Brézin, A.P.; Thulliez, P.; Wallon, Thou.; King, Fifty.; Goulet, V.; Refer, T.Northward.A.Due north. Congenital toxoplasmosis in France in 2007: First results from a national surveillance organization. Eurosurveillance 2010, fifteen, 19600. [Google Scholar] [CrossRef] [PubMed]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Brunt of 14 Pathogens in Food Sources in the United States Using Attribution Information from Outbreak Investigations and Expert Elicitation. J. Nutrient Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.; Angulo, F.; Gibb, H.; Kirk, M.; Lake, R.; et al. Attribution of global foodborne disease to specific foods: Findings from a Earth Health Organization structured expert elicitation. PLoS Ane 2017, 12, e0183641. [Google Scholar] [CrossRef]
- Pouillot, R.; Garin, B.; Ravaonindrina, Northward.; Diop, 1000.; Ratsitorahina, G.; Ramanantsoa, D.; Rocourt, J. A Risk Cess of Campylobacteriosis and Salmonellosis Linked to Chicken Meals Prepared in Households in Dakar, Senegal. Hazard Anal. 2012, 32, 1798–1819. [Google Scholar] [CrossRef]
- Nauta, M.; Christensen, B.B. The Bear upon of Consumer Phase Models in Microbial Risk Assay. Chance Anal. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Giacometti, F.; Bonilauri, P.; Albonetti, Southward.; Amatiste, S.; Arrigoni, North.; Bianchi, G.; Bertasi, B.; Bilei, South.; Bolzoni, G.; Cascone, Thou.; et al. Quantitative Risk Assessment of Human Salmonellosis and Listeriosis Related to the Consumption of Raw Milk in Italy. J. Food Prot. 2015, 78, 13–21. [Google Scholar] [CrossRef]
- Daelman, J.; Membré, J.-M.; Jacxsens, L.; Vermeulen, A.; Devlieghere, F.; Uyttendaele, Yard. A quantitative microbiological exposure assessment model for Bacillus cereus in REPFEDs. Int. J. Food Microbiol. 2013, 166, 433–449. [Google Scholar] [CrossRef]
- De Jong, A.; Verhoeff-Bakkenes, L.; Nauta, K.; De Jonge, R. Cross-contagion in the kitchen: Effect of hygiene measures. J. Appl. Microbiol. 2008, 105, 615–624. [Google Scholar] [CrossRef]
- Mylius, S.D.; Nauta, M.; Havelaar, A.H. Cross-Contamination During Food Preparation: A Mechanistic Model Applied to Craven-Borne Campylobacter. Take a chance Anal. 2007, 27, 803–813. [Google Scholar] [CrossRef]
- Verhaelen, K.; Bouwknegt, Grand.; Carratalà, A.; Lodder-Verschoor, F.; Diez-Valcarce, M.; Rodríguez-Lázaro, D.; Husman, A.M.D.R.; Rutjes, Due south.A. Virus transfer proportions between gloved fingertips, soft berries, and lettuce, and associated health risks. Int. J. Food Microbiol. 2013, 166, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Pouillot, R.; Van Doren, J.Thou.; Dennis, Due south. Reduction of Listeria monocytogenes contagion on produce—A quantitative assay of common liquid fresh produce wash compounds. Food Control 2014, 46, 430–440. [Google Scholar] [CrossRef]
- Pouillot, R.; Goulet, 5.; Delignette-Muller, M.L.; Mahé, A.; Cornu, Chiliad. Quantitative hazard cess of Listeria monocytogenes in French cold-smoked salmon: Two. Adventure characterization. Take chances Anal. 2009, 29, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Fazil, A.; Lammerding, A.M. A hazard assessment model for Escherichia coli O157:H7 in ground beef and beef cuts in Canada: Evaluating the effects of interventions. Food Control 2013, 29, 364–381. [Google Scholar] [CrossRef]
- Young, I.; Waddell, 50.; Harding, S.; Greig, J.; Mascarenhas, M.; Sivaramalingam, B.; Pham, Yard.T.; Papadopoulos, A. A systematic review and meta-assay of the effectiveness of nutrient safety education interventions for consumers in developed countries. BMC Public Health 2015, fifteen, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.B.; Hamilton, M.A.; Mitchell, E.Due west.; Kiwanuka-Tondo, J.; Fleming-Milici, F.; Proctor, D. A Meta-Assay of the Effect of Mediated Wellness Communication Campaigns on Behavior Change in the United States. J. Health Commun. 2004, nine, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Anker, A.E.; Feeley, T.H.; McCracken, B.; Lagoe, C.A. Measuring the Effectiveness of Mass-Mediated Health Campaigns through Meta-Assay. J. Wellness Commun. 2016, 21, 439–456. [Google Scholar] [CrossRef]
- ANSES. Stance of xiv October 2015 of the French Bureau for Nutrient, Environmental and Occupational Health & Safety Relating to Consumer Information on Prevention of Foodborne Microbiological Risks. 2015. Available online: https://world wide web.anses.fr/en/system/files/BIORISK2012sa0118Ra-02EN.pdf (accessed on 28 August 2020).
- Havelaar, A.H.; Galindo, Á.V.; Kurowicka, D.; Cooke, R. Attribution of Foodborne Pathogens Using Structured Expert Elicitation. Foodborne Pathog. Dis. 2008, 5, 649–659. [Google Scholar] [CrossRef]
- Greig, J.; Ravel, A. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Nutrient Microbiol. 2009, 130, 77–87. [Google Scholar] [CrossRef]
- Ravel, A.; Greig, J.; Tinga, C.; Todd, Due east.; Campbell, K.; Cassidy, One thousand.; Marshall, B.; Pollari, F. Exploring Historical Canadian Foodborne Outbreak Information Sets for Human Illness Attribution. J. Food Prot. 2009, 72, 1963–1976. [Google Scholar] [CrossRef]
- EFSA. Console on Biological Hazards, Scientific stance on the risks for public wellness related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, fourteen, 93. [Google Scholar]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, Southward.; De Zutter, 50.; Daube, Thousand.; Sindic, M.; Uyttendaele, K.; Herman, Fifty. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015, fifty, 32–44. [Google Scholar] [CrossRef]
- Mullner, P.; Spencer, S.Due east.; Wilson, D.J.; Jones, G.; Noble, A.D.; Midwinter, A.C.; Collins-Emerson, J.Thou.; Carter, P.; Hathaway, S.; French, N.P. Assigning the source of human campylobacteriosis in New Zealand: A comparative genetic and epidemiological arroyo. Infect. Genet. Evol. 2009, nine, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Gras, 50.M.; Smid, J.H.; Wagenaar, J.A.; De Boer, A.G.; Havelaar, A.H.; Friesema, I.H.G.; French, N.P.; Busani, L.; Van Pelt, Westward. Risk Factors for Campylobacteriosis of Chicken, Ruminant, and Environmental Origin: A Combined Example-Command and Source Attribution Analysis. PLoS Ane 2012, 7, e42599. [Google Scholar] [CrossRef]
- Heusinkveld, M.; Mughini-Gras, L.; Pijnacker, R.; Vennema, H.; Scholts, R.; Van Huisstede-Vlaanderen, K.W.; Kortbeek, T.; Kooistra-Smid, M.; Van Pelt, W. Potential causative agents of acute gastroenteritis in households with preschool children: Prevalence, gamble factors, clinical relevance and household transmission. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1691–1700. [Google Scholar] [CrossRef]
- Peck, M.W.; Stringer, Due south.C.; Carter, A.T. Clostridium botulinum in the mail service-genomic era. Food Microbiol. 2011, 28, 183–191. [Google Scholar] [CrossRef]
- Carter, A.T.; Peck, M.Due west. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res. Microbiol. 2015, 166, 303–317. [Google Scholar] [CrossRef]
- Male monarch, L.-A.; Popoff, M.-R.; Mazuet, C.; Espié, E.; Vaillant, V.; De Valk, H. Le botulisme infantile en France, 1991–2009. Curvation. Pédiatrie 2010, 17, 1288–1292. [Google Scholar] [CrossRef]
- Hoffmann, S.; Fischbeck, P.; Krupnick, A.; McWilliams, G. Using Skilful Elicitation to Link Foodborne Illnesses in the United States to Foods. J. Food Prot. 2007, 70, 1220–1229. [Google Scholar] [CrossRef]
- Smith, J.L. Shigella as a Nutrient borne Pathogen. J. Nutrient Prot. 1987, l, 788–801. [Google Scholar] [CrossRef]
- Skovgaard, N. Risk assessment of Vibrio parahaemolyticus in seafood. Interpretative Summary and Technical Study. Int. J. Food Microbiol. 2012, 154, 215–216. [Google Scholar] [CrossRef]
- Le Guern, A.-Southward.; Martin, L.; Savin, C.; Carniel, E. Yersiniosis in France: Overview and potential sources of infection. Int. J. Infect. Dis. 2016, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, Ten.-J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Veter Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance, fourth ed. 2020. Available online: https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls (accessed on 10 Nov 2020).
- Erickson, M.C.; Ortega, Y.R. Inactivation of Protozoan Parasites in Food, Water, and Ecology Systems. J. Nutrient Prot. 2006, 69, 2786–2808. [Google Scholar] [CrossRef]
- Federer, Thou.; Armua-Fernandez, One thousand.T.; Hoby, Due south.; Wenker, C.; Deplazes, P. In vivo viability of Echinococcus multilocularis eggs in a rodent model after different thermo-treatments. Exp. Parasitol. 2015, 154, 14–nineteen. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.; Valero, Thousand. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 2005, 35, 1255–1278. [Google Scholar] [CrossRef]
- Nutrient and Agriculture System of the United nations/Globe Health Organization. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites; Microbiological Risk Assessment Series No. 23; FAO: Rome, Italia, 2014; 302p. [Google Scholar]
- Fritz, H.; Barr, B.; Packham, A.; Melli, A.; Conrad, P. Methods to produce and safely piece of work with large numbers of Toxoplasma gondii oocysts and bradyzoite cysts. J. Microbiol. Methods 2012, 88, 47–52. [Google Scholar] [CrossRef]
- Dupouy-Camet, J. Trichinellosis: A worldwide zoonosis. Veter Parasitol. 2000, 93, 191–200. [Google Scholar] [CrossRef]
Effigy i. Sankey diagram of the foodborne disease burden co-ordinate to the unlike food categories and the consumers practices (detailed percentages are given in Table five).
Figure ane. Sankey diagram of the foodborne disease burden according to the different food categories and the consumers practices (detailed percentages are given in Table 5).
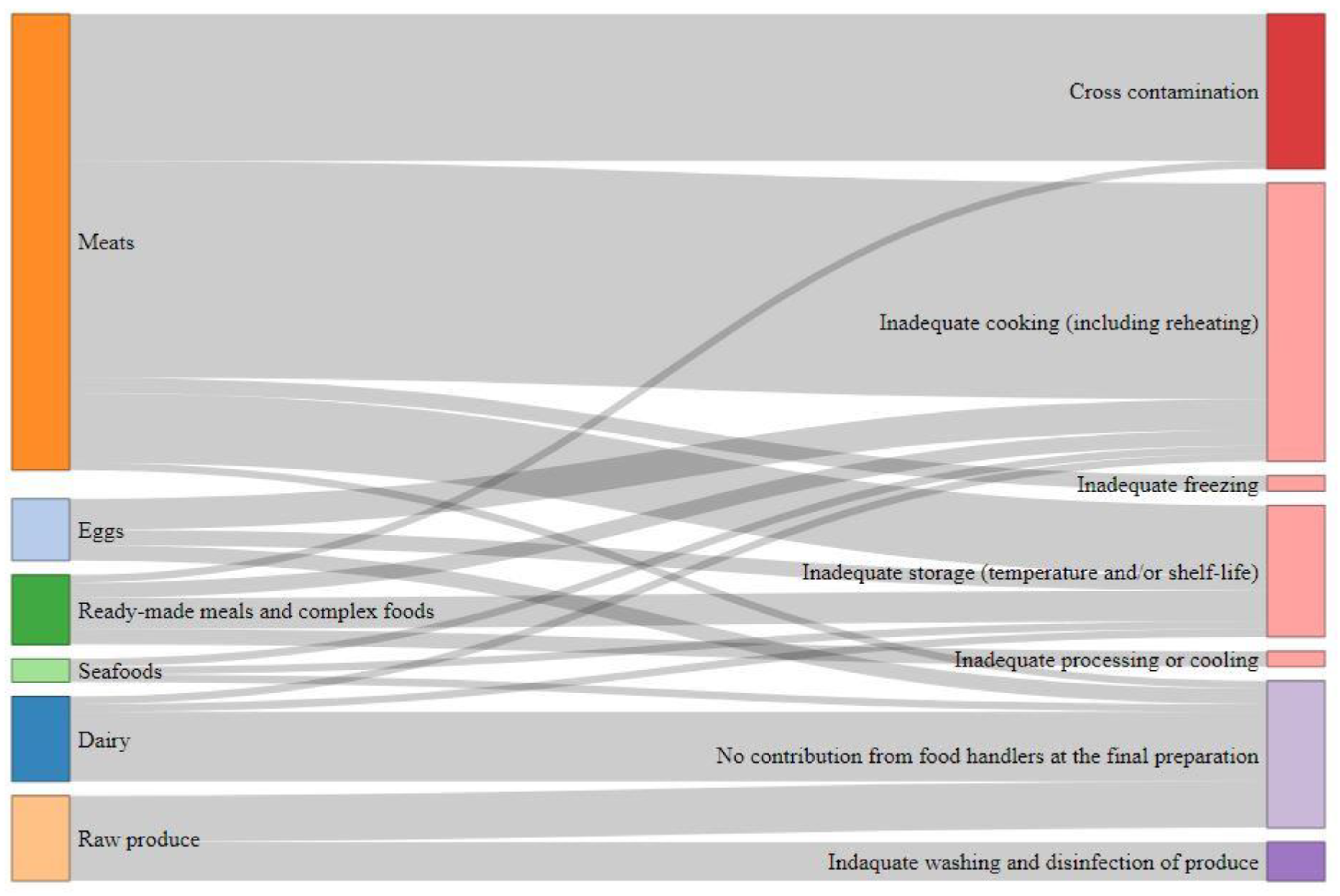
Table 1. Incidence of foodborne illnesses in France (circa 2010): estimated incidence, mean incidence rate of cases per 100,000 persons and attributed scores.
Table 1. Incidence of foodborne illnesses in France (circa 2010): estimated incidence, mean incidence rate of cases per 100,000 persons and attributed scores.
| Hazards | Incidence | Incidence Rate | References | Scores | |
|---|---|---|---|---|---|
| Bacteria, toxins and metabolites | Bacillus cereus | 69,000 | 110 | Van Cauteren et al. [i] | 5 |
| Brucella spp. | 24 | 0.04 | Mailles et al. [22] | 1 | |
| Campylobacter spp. | 390,000 | 600 | Van Cauteren et al. [i] | 5 | |
| Clostridium botulinum | 21 | 0.03 | Van Cauteren et al. [ane] | 1 | |
| Clostridium perfringens | 120,000 | 180 | Van Cauteren et al. [1] | v | |
| Histamine | 167 | 0.3 | Santé Publique France [23] | 2 | |
| Listeria monocytogenes | 400 | 0.6 | Van Cauteren et al. [1] | two | |
| Salmonella not-typhoidal | 180,000 | 280 | Van Cauteren et al. [i] | 5 | |
| Shiga toxin-producing East. coli (STEC) | 18,000 | 28 | Van Cauteren et al. [1] | 4 | |
| Shigella spp. | 3400 | 5.ii | Van Cauteren et al. [i] | iii | |
| Staphylococcus aureus | 73,000 | 110 | Van Cauteren et al. [1] | 5 | |
| Vibrio parahaemolyticus | 5 | 0.008 | NRC Vibrio [24] | 0 | |
| Yersinia enterocolitica | 21,000 | 32 | Van Cauteren et al. [1] | 4 | |
| Viruses | Hepatitis A virus | 2600 | 4.0 | Van Cauteren et al. [1] | 3 |
| Hepatitis Due east virus | 2300 | three.five | NRC HEV [25] | 3 | |
| Norovirus | 520,000 | 800 | Van Cauteren et al. [1] | 5 | |
| Parasites | Anisakis spp. | eight | 0.01 | Van Cauteren et al. [fifteen] | 1 |
| Cryptosporidium spp. | 101 | 0.ii | NRC Cryptosporidium [26] | ii | |
| Cyclospora cayetanensis | 8 | 0.01 | Medical network Anofel [26] | 1 | |
| Echinococcus multilocularis | 29 | 0.04 | Van Cauteren et al. [xv] | 1 | |
| Fasciola hepatica | five | 0.008 | Van Cauteren et al. [fifteen] | 0 | |
| Giardia spp. | 482 | 0.vii | Medical network Anofel [26] | ii | |
| Taenia saginata | 33,000 | 51 | Van Cauteren et al. [1] | four | |
| Toxoplasma gondii, acquired | eleven,500 | xviii | Van Cauteren et al. [1] | iv | |
| Toxoplasma gondii, congenital | 300 | 0.5 | Van Cauteren et al. [ane] | ii | |
| Trichinella spp. | 11 | 0.02 | Van Cauteren [27] | ane | |
Table 2. Severity scores of foodborne illnesses (based on median foodborne disability-adapted life years (DALYs) per k cases of illness) and estimated foodborne disease burden in French republic (circa 2010).
Table 2. Severity scores of foodborne illnesses (based on median foodborne inability-adjusted life years (DALYs) per grand cases of illness) and estimated foodborne disease burden in France (circa 2010).
| Hazards | Severity | Incidence | Foodborne Disease Burden (FBDB) | ||||
|---|---|---|---|---|---|---|---|
| The netherlands, 2009 [17] | EUR A, 2010 [xviii,nineteen] | This Written report | |||||
| DALYs | DALYs | Score | Score | Score | % of Total FBDB | ||
| Bacteria, toxins and metabolites | Bacillus cereus | 2.iii | - | 1 | five | 6 | 3% |
| Brucella spp. | - | 300 | iii | one | 4 | 0.03% | |
| Campylobacter spp. | 41 | twenty | 2 | 5 | 7 | 32% | |
| Clostridium botulinum | - | - | three | 1 | four | 0.03% | |
| Clostridium perfringens | iii.2 | - | 1 | 5 | 6 | 3% | |
| Histamine | - | - | i | two | 3 | 0.003% | |
| Listeria monocytogenes | 1450 | 8000 | 4 | 2 | half-dozen | three% | |
| Salmonella, not-typhoidal | 49 | seventy | 2 | v | 7 | 32% | |
| Shiga toxin-producing East. coli | 143 1 | 20 | 2 | 4 | 6 | 3% | |
| Shigella spp. | - | 70 | 2 | 3 | five | 0.three% | |
| Staphylococcus aureus | 2.half-dozen | - | one | 5 | vi | 3% | |
| Vibrio parahaemolyticus | - | - | ane | 0 | 1 | 0.00003% | |
| Yersinia enterocolitica | - | - | 2 | 4 | 6 | three% | |
| Virus | Hepatitis A virus | 167 | 100 | 3 | three | 6 | 3% |
| Hepatitis E virus | 460 | - | 3 | 3 | 6 | 3% | |
| Norovirus | ii.iv | 2 | 1 | 5 | 6 | iii% | |
| Parasites | Anisakis spp. | - | - | i | 1 | 2 | 0.0003% |
| Cryptosporidium spp. | 2.9 | 8 | 1 | 2 | 3 | 0.003% | |
| Cyclospora cayentanensis | - | - | 1 | i | 2 | 0.0003% | |
| Echinococcus multilocularis | - | 2000 | four | 1 | v | 0.3% | |
| Fasciola hepatica | - | 9000 | 2 | 0 | 2 | 0.0003% | |
| Giardia spp. | 2.1 | 1 | 1 | two | 3 | 0.003% | |
| Taenia saginata | - | - | 1 | iv | 5 | 0.3% | |
| Toxoplasma gondii, acquired | 3170 | 60 | ii | 4 | 6 | 3% | |
| Toxoplasma gondii, built | 6360 | 6300 | iv | 2 | 6 | three% | |
| Trichinella spp. | - | 100 | 2 | ane | three | 0.003% | |
Tabular array 3. Foodborne disease burden (xc% incertitude intervals) for exposure routes and food-handling practices implicated in the transmission of biological foodborne hazards.
Tabular array 3. Foodborne disease brunt (90% incertitude intervals) for exposure routes and food-handling practices implicated in the manual of biological foodborne hazards.
| Food Categories | Sub-Categories | Specific Foods | Hazards | Default in Handling Practices Contributing to the Onset of Foodborne Illnesses | No Contribution from Food Handlers at the Concluding Preparation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cross Contagion | Inadequate Washing and Disinfection of Produce | Inadequate Processing or Cooling | Inadequate Freezing | Inadequate Cooking (Including Reheating) | Inadequate Storage (Temperature and/or Shelf-Life) | |||||
| Meats | Beefiness | Cooked basis beef | STEC | - | - | - | - | (0.2–one.viii) | - | - |
| Salmonella | - | - | - | - | (0.2–half dozen.6) | (0.2–6.six) | - | |||
| Raw ground beef | STEC | - | - | - | - | - | (0.2–1.eight) | |||
| Salmonella | - | - | - | - | - | (0.ii–6.half dozen) | (0.2–6.6) | |||
| Beef meat | T. saginata | - | - | (0.0–0.iii) | (0.0–0.three) | - | - | |||
| Poultry | Poultry meat | Campylobacter spp. | (3–29) | - | - | - | (3–29) | - | - | |
| Salmonella | (0.1–iv.seven) | - | - | - | (0.1–4.7) | (0.1–4.seven) | - | |||
| Pork | Pork meat | Salmonella | (0.1–4.7) | - | - | - | (0.1–4.seven) | (0.1–4.7) | - | |
| Y. enterocolitica | (0.2–two.v) | - | - | - | (0.ii–2.5) | (0.two–2.v) | - | |||
| Raw hog liver products, boar offal | Hepatitis E virus | - | - | - | - | 3.2 | - | - | ||
| Open-air pig, boar, game | Trichinella spp. | - | - | - | (0.000–0.003) | (0.000–0.003) | - | - | ||
| Cooked pork meats | Fifty. monocytogenes | - | - | - | - | - | (0.0–0.9) | (0.0–0.9) | ||
| Due south. aureus | - | - | - | - | - | (0.two–ii.5) | - | |||
| Home-made cooked and salt pork meat | C. botulinum | - | - | (0.00–0.02) | - | - | (0.00–0.02) | - | ||
| Other meats | Lamb, open-air pigs, imported horses | T. gondii, built | - | - | - | (0.2–2.1) | (0.ii–two.one) | - | - | |
| T. gondii, acquired | - | - | - | (0.2–two.i) | (0.ii–2.1) | - | - | |||
| Dairy | Unpasteurised milk | Heated milk | Brucella spp. | - | - | - | - | (0.00–0.03) | - | - |
| STEC | - | - | - | - | (0.2–1.8) | - | - | |||
| Raw milk | Brucella spp. | - | - | - | - | - | - | (0.00–0.03) | ||
| STEC | - | - | - | - | - | - | (0.2–1.viii) | |||
| Raw milk cheeses | Fresh non-ripened cheeses | Brucella spp. | - | - | - | - | - | - | (0.00–0.03) | |
| Hard non-cooked cheeses | Salmonella | - | - | - | - | - | - | (1.6–12.5) | ||
| Soft cheeses | STEC | - | - | - | - | - | - | (0.two–one.8) | ||
| Salmonella | - | - | - | - | - | - | (ane.half-dozen–12.5) | |||
| S. aureus | - | - | - | - | - | - | (0.2–two.5) | |||
| L. monocytogenes | - | - | - | - | - | (0.0–0.9) | (0.0–0.9) | |||
| Pasteurized milk cheeses | Soft cheeses | L. monocytogenes | - | - | - | - | - | (0.0–0.9) | (0.0–0.9) | |
| Eggs | - | Eggs | Salmonella | - | - | - | - | (one.6–12.5) | - | - |
| Raw eggs products | Salmonella | - | - | - | - | - | (0.2–6.viii) | (0.ii–half dozen.8) | ||
| Seafood | Fish | Cooked fish | Anisakis spp. | - | - | (0–0.0002) | (0–0.0002) | (0–0.0002) | - | - |
| Raw fish | Anisakis spp. | - | - | (0–0.0002) | (0–0.0002) | - | - | - | ||
| Cold smoked fish | L. monocytogenes | - | - | - | - | - | (0.0–0.9) | (0.0–0.9) | ||
| Fish species with a loftier amount of histidine (particularly tuna) | Histamine | - | - | - | - | - | - | 0.03 | ||
| Shellfish | Crustaceans | V. parahaemolyticus | - | - | - | - | (0–0.00003) | - | - | |
| Fifty. monocytogenes | - | - | - | - | - | (0.0–0.9) | (0.0–0.ix) | |||
| Cooked bivalve mollusks | V. parahaemolyticus | - | - | - | - | (0–0.00003) | - | - | ||
| Norovirus | - | - | - | - | (0.2–2.0) | - | - | |||
| Hepatitis A virus | - | - | - | - | (0.ii–ii.0) | - | - | |||
| Raw bivalve mollusks | V. parahaemolyticus | - | - | - | - | - | (0–0.00003) | (0–0.00003) | ||
| Norovirus | - | - | - | - | - | - | (0.2–two.0) | |||
| Hepatitis A virus | - | - | - | - | - | - | (0.2–2.0) | |||
| Raw produce | - | Frozen raw produce (crimson fruits, vegetables) | Norovirus | - | - | - | - | - | - | (0.two–2.0) |
| Hepatitis A virus | - | - | - | - | - | - | (0.2–ii.0) | |||
| Not frozen raw produce | STEC | - | (0.two–1.8) | - | - | - | - | |||
| Fifty. monocytogenes | - | (0.0–0.6) | - | - | - | (0.0–0.6) | (0.0–0.half-dozen) | |||
| Salmonella | - | (1.6–12.5) | - | - | - | - | ||||
| Norovirus | - | - | - | - | - | - | (0.two–2.0) | |||
| Hepatitis A virus | - | - | - | - | - | - | (0.2–two.0) | |||
| Cryptosporidium spp. | - | 0.003 | - | - | - | - | ||||
| C. cayetanensis | - | - | - | - | - | - | 0.0003 | |||
| Giardia spp. | - | 0.003 | - | - | - | - | ||||
| T. gondii, congenital | - | - | - | - | - | - | (0.three–2.9) | |||
| T. gondii, acquired | - | - | - | - | - | - | (0.iii–2.9) | |||
| Wild raw produce (watercress, dandelion) | F. hepatica | - | - | - | - | - | - | 0.0003 | ||
| Crimson fruits and berries | E. multilocularis | - | - | - | - | (0.0–0.3) | - | (0.0–0.3) | ||
| Ready-fabricated meals and complex foods | Refrigerated and processed foods of extended immovability (REPFED) | All kinds of packaging | B. cereus | - | - | - | - | - | (0.3–2.9) | - |
| Vacuum-packed | C. botulinum | - | - | - | - | (0.00–0.02) | (0.00–0.02) | - | ||
| Home-made meal | Particularly those containing grain (pasta, rice, semolina) or dehydrated ingredients | B. cereus | - | - | (0.0–1.6) | - | (0.0–1.6) | (0.0–1.6) | - | |
| Specially meat cooked in a sauce | C. perfringens | - | - | (0.2–2.v) | - | (0.2–2.5) | (0.2–2.5) | - | ||
| Composite foods | Ready-fabricated meals, cakes, extensively manipulated foods (sandwiches) | L. monocytogenes | - | - | - | - | - | (0.0–0.nine) | (0.0–0.9) | |
| S. aureus | - | - | (0.1–1.6) | - | - | (0.one–1.half-dozen) | - | |||
| Shigella spp. | 0.2 (0.0–0.3) | - | - | - | - | - | (0.0–0.3) | |||
| Norovirus | 0.six (0.2–2.0) | - | - | - | - | - | - | |||
| Hepatitis A virus | 0.six (0.2–2.0) | - | - | - | - | - | - | |||
| Habitation-made canned food | C. botulinum | - | - | (0.00–0.02) | - | (0.00–0.02) | - | - | ||
Tabular array 4. Attribution of foodborne disease brunt (FBDB) to foods.
Tabular array 4. Attribution of foodborne disease burden (FBDB) to foods.
| Nutrient Categories | CI90 1 (%) | Sub-Categories | CI90 (%) | Specific Foods | CI90 (%) |
|---|---|---|---|---|---|
| Meats | (50–69) | Beef | (4–20) | Cooked ground beef | (2–13) |
| Raw ground beef | (two–xiii) | ||||
| Beefiness meat | 0.3 | ||||
| Poultry | (34–44) | Poultry meat | (34–44) | ||
| Pork | (9–21) | Pork meat | (5–sixteen) | ||
| Raw pig liver products, boar offal | 3 | ||||
| Open up-air sus scrofa, boar, game | 0.003 | ||||
| Cooked pork meats | (0–3) | ||||
| Home-fabricated cooked and salt pork meat | (0–0.03) | ||||
| Other meats | (i–5) | Lamb, open up-air pigs, imported horses | (ane–v) | ||
| Dairy | (5–22) | Unpasteurised milk | (0–2) | Heated milk | (0–2) |
| Raw milk | (0–2) | ||||
| Raw milk cheeses | (4–21) | Fresh non-ripened cheeses | (0–0.03) | ||
| Difficult non-cooked cheeses | (2–12) | ||||
| Soft cheeses | (2–fifteen) | ||||
| Pasteurized milk cheeses | (0.ii–one.six) | Soft cheeses | (0.2–one.6) | ||
| Eggs | (3–nineteen) | - | - | Eggs | (ii–13) |
| Raw eggs products | (two–13) | ||||
| Seafood | (one–6) | Fish | (0.two–1.half dozen) | Cooked fish | (0–0.0003) |
| Raw fish | (0–0.0003) | ||||
| Cold smoked fish | (0.two–1.half dozen) | ||||
| Fish species with a loftier amount of histidine (specially tuna) | 0.003 | ||||
| Shellfish | (1–5) | Crustaceans | (0.2–1.half dozen) | ||
| Cooked bivalve mollusks | (0–three) | ||||
| Raw bivalve mollusks | (0–3) | ||||
| Raw produce | (6–20) | - | - | Frozen raw produce (ruby-red fruits, vegetables) | (0–three) |
| Not frozen raw produce | (5–eighteen) | ||||
| Wild raw produce (watercress, dandelion) | 0.0003 | ||||
| Red fruits and berries | 0.3 | ||||
| Ready-fabricated meals and circuitous foods | (8–12) | Refrigerated and candy foods of extended immovability (REPFED) | (0–iii) | All kinds of packaging | (0–3) |
| Vacuum-packed | (0–0.02) | ||||
| Home-made meal | (3–6) | Particularly those containing grain (pasta, rice, semolina) or dehydrated ingredients | (0–three) | ||
| Particularly meat cooked in a sauce | 3 | ||||
| Composite foods | (1–5) | Prepare-fabricated meals, cakes, extensively manipulated foods (sandwiches) | (ane–5) | ||
| Abode-made canned nutrient | (0–0.03) |
Table 5. Attribution of foodborne affliction burden (FBDB) to nutrient-treatment practices.
Table five. Attribution of foodborne disease burden (FBDB) to food-handling practices.
| Food Categories | CI90 one (%) | Poor Handling Practices Contributing to the Onset of Foodborne Illnesses | No Contribution from Nutrient Handlers at the Final Preparation | |||||
|---|---|---|---|---|---|---|---|---|
| Cross-Contagion | Inadequate Washing and Disinfection of Produce | Inadequate Processing or Cooling | Inadequate Freezing | Inadequate Cooking (Including Reheating) | Inadequate Storage (Temperature and/or Shelf-Life) | |||
| Meats | (50–69) | (6–33) | 0 | (0.00–0.02) | (one–3) | (13–41) | (3–fifteen) | (i–vii) |
| Dairy | (5–22) | 0 | 0 | 0 | 0 | (0–2) | (0.1–1.4) | (five–21) |
| Eggs | (3–19) | 0 | 0 | 0 | 0 | (2–13) | (0–vii) | (0–7) |
| Seafoods | (ane–six) | 0 | 0 | (0–0.0002) | (0–0.0002) | (0–3) | (0.1–1.4) | (1–three) |
| Raw produce | (half-dozen–20) | 0 | (2–13) | 0 | 0 | 0 | (0.0–0.6) | (3–9) |
| Ready-fabricated meals and complex foods | (8–12) | (0–iii) | 0 | (1–4) | 0 | (0–3) | (2–six) | (0.ane–1.0) |
| Total | - | (7–34) | (2–13) | (i–4) | (i–3) | (19–49) | (9–23) | (15–33) |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open admission commodity distributed under the terms and weather of the Artistic Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/iv.0/).
Source: https://www.mdpi.com/2304-8158/9/11/1644/htm
ارسال یک نظر for "Food Illness Caused by Ground Beef 911"