what is the ratio of al ions to s ions in a neutral compound?
Chapter 3. Atoms, Molecules, and Ions
Ions and Ionic Compounds
- Know how ions course.
- Larn the characteristic charges that ions have.
- Construct a proper formula for an ionic chemical compound.
- Generate a proper proper noun for an ionic compound.
So far, nosotros accept discussed elements and compounds that are electrically neutral. They take the aforementioned number of electrons as protons, so the negative charges of the electrons is counterbalanced by the positive charges of the protons. Nonetheless, this is not ever the example. Electrons tin move from one atom to some other; when they do, species with overall electric charges are formed. Such species are called . Species with overall positive charges are termed , while species with overall negative charges are called . Remember that ions are formed only when electrons move from one cantlet to another; a proton never moves from 1 atom to some other. Compounds formed from positive and negative ions are chosen .
Individual atoms can gain or lose electrons. When they do, they become monatomic ions. When atoms gain or lose electrons, they commonly gain or lose a feature number of electrons and and then take on a characteristic overall charge. Tabular array 3.2 "Monatomic Ions of Various Charges" lists some common ions in terms of how many electrons they lose (making cations) or gain (making anions). There are several things to notice about the ions in Table three.2. First, each element that forms cations is a metal, except for 1 (hydrogen), while each element that forms anions is a nonmetal. This is actually one of the chemical properties of metals and nonmetals: metals tend to form cations, while nonmetals tend to course anions. Second, most atoms form ions of a single characteristic charge. When sodium atoms form ions, they e'er form a 1+ charge, never a 2+ or 3+ or fifty-fifty 1− accuse. Thus, if y'all commit the information in Table 3.2 to memory, you volition always know what charges most atoms form. (In Chapter 9 "Chemical Bonds", we will hash out why atoms class the charges they do.)
| Ions formed by losing a unmarried electron | H+ Na+ K+ Rb+ Ag+ Au+ |
|---|---|
| Ions formed past losing two electrons | Mgtwo+ Caii+ Sr2+ Fe2+ Coii+ Ni2+ Cu2+ Zn2+ Sntwo+ Hgii+ Atomic number 822+ |
| Ions formed by losing three electrons | Sc3+ Iron3+ Cothree+ Niiii+ Authree+ Al3+ Cr3+ |
| Ions formed by losing four electrons | Tifour+ Sn4+ Pbiv+ |
| Ions formed by gaining a unmarried electron | F− Cl− Br− I− |
| Ions formed by gaining two electrons | O2− Sii− Se2− |
| Ions formed by gaining iii electrons | N3− P3− |
Third, there are some exceptions to the previous point. A few elements, all metals, tin can form more than one possible accuse. For case, fe atoms can form 2+ cations or 3+ cations. Cobalt is another chemical element that can form more than than one possible charged ion (ii+ and 3+), while atomic number 82 can class 2+ or 4+ cations. Unfortunately, there is little understanding which two charges a metal atom may take, and so it is all-time to just memorize the possible charges a particular element can have.
Note the convention for indicating an ion. The magnitude of the charge is listed as a correct superscript side by side to the symbol of the chemical element. If the charge is a unmarried positive or negative one, the number ane is non written; if the magnitude of the charge is greater than ane, and then the number is written before the + or − sign. An element symbol without a accuse written adjacent to information technology is assumed to exist the uncharged atom.
Naming an ion is straightforward. For a cation, only use the name of the chemical element and add together the word ion (or if you lot want to be more specific, add cation) after the element's name. And then Na+ is the sodium ion; Ca2+ is the calcium ion. If the chemical element has more than ane possible charge, the value of the charge comes after the element name and before the word ion. Thus, Fe2+ is the fe two ion, while Fethree+ is the atomic number 26 three ion. In impress, we use roman numerals in parentheses to represent the charge on the ion, so these 2 iron ions would be represented as the iron(II) cation and the fe(III) cation, respectively.
For a monatomic anion, use the stalk of the element proper name and suspend the suffix -ide to information technology, then add ion. This is like to how we named molecular compounds. Thus, Cl− is the chloride ion, and N3− is the nitride ion.
Problems
Name each species.
- Oii−
- Co
- Coii+
Solutions
- This species has a 2− charge on information technology, so it is an anion. Anions are named using the stalk of the chemical element name with the suffix -ide added. This is the oxide anion.
- Because this species has no accuse, it is an atom in its elemental form. This is cobalt.
- In this case, there is a 2+ charge on the cantlet, so it is a cation. We note from Table 3.2 "Monatomic Ions of Diverse Charges" that cobalt cations tin have two possible charges, and then the name of the ion must specify which charge the ion has. This is the cobalt(Two) cation.
Examination Yourself
Name each species.
- Pthree−
- Srii+
Answers
- the phosphide anion
- the strontium cation
Chemical formulas for ionic compounds are called ionic formulas. A proper ionic formula has a cation and an anion in it; an ionic compound is never formed between two cations only or ii anions only. The key to writing proper ionic formulas is simple: the total positive charge must residual the full negative charge. Considering the charges on the ions are feature, sometimes nosotros have to have more than than one of a cation or an anion to remainder the overall positive and negative charges. It is conventional to use the lowest ratio of ions that are needed to residual the charges.
For example, consider the ionic compound between Na+ and Cl−. Each ion has a single charge, one positive and one negative, so nosotros demand only one ion of each to rest the overall charge. When writing the ionic formula, we follow two additional conventions: (1) write the formula for the cation first and the formula for the anion next, merely (ii) do not write the charges on the ions. Thus, for the compound between Na+ and Cl−, we have the ionic formula NaCl (Figure 3.one "NaCl = Table Table salt"). The formula Na2Cl2 also has counterbalanced charges, but the convention is to use the lowest ratio of ions, which would be i of each. (Call back from our conventions for writing formulas that we don't write a 1 subscript if there is only one atom of a particular element present.) For the ionic compound between magnesium cations (Mg2+) and oxide anions (O2−), once more nosotros need just one of each ion to balance the charges. By convention, the formula is MgO.

For the ionic compound between Mg2+ ions and Cl− ions, we at present consider the fact that the charges have different magnitudes, 2+ on the magnesium ion and one− on the chloride ion. To remainder the charges with the lowest number of ions possible, we demand to have two chloride ions to balance the accuse on the one magnesium ion. Rather than write the formula MgClCl, we combine the two chloride ions and write it with a 2 subscript: MgCl2.
What is the formula MgClii telling us? There are ii chloride ions in the formula. Although chlorine equally an element is a diatomic molecule, Cl2, elemental chlorine is not part of this ionic compound. The chlorine is in the form of a negatively charged ion, not the neutral element. The 2 subscript is in the ionic formula because we need two Cl− ions to balance the charge on 1 Mg2+ ion.
Bug
Write the proper ionic formula for each of the two given ions.
- Ca2+ and Cl−
- Aliii+ and F−
- Aliii+ and O2−
Solutions
- We need 2 Cl− ions to balance the charge on 1 Caii+ ion, so the proper ionic formula is CaClii.
- We demand three F− ions to balance the charge on the Aliii+ ion, and so the proper ionic formula is AlFthree.
- With Al3+ and Otwo−, note that neither charge is a perfect multiple of the other. This means we have to become to a least mutual multiple, which in this example will be six. To get a total of 6+, we need ii Al3+ ions; to get 6−, we demand three O2− ions. Hence the proper ionic formula is AltwoO3.
Test Yourself
Write the proper ionic formulas for each of the ii given ions.
- Fe2+ and Stwo−
- Fe3+ and South2−
Answers
- FeS
- Fe2South3
Naming ionic compounds is simple: combine the name of the cation and the proper name of the anion, in both cases omitting the word ion. Practise not utilise numerical prefixes if at that place is more than one ion necessary to balance the charges. NaCl is sodium chloride, a combination of the proper noun of the cation (sodium) and the anion (chloride). MgO is magnesium oxide. MgCl2 is magnesium chloride—non magnesium dichloride.
In naming ionic compounds whose cations tin take more than ane possible charge, we must also include the charge, in parentheses and in roman numerals, every bit part of the proper name. Hence FeS is iron(Ii) sulfide, while Fe2S3 is iron(III) sulfide. Once more, no numerical prefixes appear in the name. The number of ions in the formula is dictated past the need to residual the positive and negative charges.
Problems
Proper name each ionic compound.
- CaCltwo
- AlFiii
- CotwoO3
Solutions
- Using the names of the ions, this ionic compound is named calcium chloride. It is not calcium(Ii) chloride considering calcium forms but 1 cation when it forms an ion, and it has a characteristic charge of 2+.
- The name of this ionic compound is aluminum fluoride.
- Nosotros know that cobalt can have more than one possible charge; we simply need to determine what it is. Oxide always has a ii− charge, so with 3 oxide ions, we have a total negative charge of 6−. This ways that the two cobalt ions have to contribute 6+, which for two cobalt ions means that each i is 3+. Therefore, the proper name for this ionic compound is cobalt(III) oxide.
Test Yourself
Name each ionic compound.
- Sc2Oiii
- AgCl
Answers
- scandium oxide
- silvery chloride
How practice y'all know whether a formula—and by extension, a name—is for a molecular chemical compound or for an ionic compound? Molecular compounds form betwixt nonmetals and nonmetals, while ionic compounds class between metals and nonmetals. The periodic tabular array (Effigy 3.5 "A Simple Periodic Table") can be used to make up one's mind which elements are metals and nonmetals.
In that location also exists a group of ions that comprise more than 1 atom. These are called polyatomic ions. Table three.three "Common Polyatomic Ions" lists the formulas, charges, and names of some common polyatomic ions. Just one of them, the ammonium ion, is a cation; the rest are anions. Well-nigh of them likewise contain oxygen atoms, so sometimes they are referred to equally oxyanions. Some of them, such as nitrate and nitrite, and sulfate and sulfite, have very similar formulas and names, so care must be taken to get the formulas and names correct. Annotation that the -ite polyatomic ion has one less oxygen atom in its formula than the -ate ion but with the aforementioned ionic charge.
| Name | Formula and Charge |
|---|---|
| ammonium | NHiv + |
| acetate | C2HiiiO2 −, or CH3COO− |
| bicarbonate (hydrogen carbonate) | HCO3 − |
| bisulfate (hydrogen sulfate) | HSO4 − |
| carbonate | CO3 ii− |
| chlorate | ClOthree − |
| chromate | CrO4 ii− |
| cyanide | CN− |
| dichromate | Cr2O7 2− |
| hydroxide | OH− |
| nitrate | NOiii − |
| nitrite | NO2 − |
| peroxide | O2 2− |
| perchlorate | ClO4 − |
| phosphate | PO4 three− |
| sulfate | SO4 two− |
| sulfite | Then3 2− |
| triiodide | I3 − |
The naming of ionic compounds that comprise polyatomic ions follows the same rules equally the naming for other ionic compounds: but combine the name of the cation and the name of the anion. Exercise not utilise numerical prefixes in the name if there is more than than 1 polyatomic ion; the only exception to this is if the name of the ion itself contains a numerical prefix, such every bit dichromate or triiodide.
Writing the formulas of ionic compounds has i important difference. If more than ane polyatomic ion is needed to residuum the overall charge in the formula, enclose the formula of the polyatomic ion in parentheses and write the proper numerical subscript to the right and outside the parentheses. Thus, the formula betwixt calcium ions, Caii+, and nitrate ions, NOthree −, is properly written Ca(NOthree)ii, not CaNO32 or CaN2Ohalf-dozen. Apply parentheses where required. The name of this ionic compound is simply calcium nitrate.
Problems
Write the proper formula and give the proper proper name for each ionic chemical compound formed between the two listed ions.
- NH4+ and Southwardii−
- Al3+ and POfour 3−
- Fe2+ and PO4 3−
Solutions
- Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we demand two ammonium ions to balance the charge on a single sulfide ion. Enclosing the formula for the ammonium ion in parentheses, we have (NH4)2South. The compound's name is ammonium sulfide.
- Considering the ions have the same magnitude of charge, nosotros demand only ane of each to residue the charges. The formula is AlPO4, and the name of the compound is aluminum phosphate.
- Neither charge is an exact multiple of the other, so we have to become to the least common multiple of 6. To go 6+, nosotros need three iron(Two) ions, and to get six−, we need two phosphate ions. The proper formula is Fe3(PO4)2, and the compound's name is iron(2) phosphate.
Test Yourself
Write the proper formula and give the proper name for each ionic chemical compound formed between the two listed ions.
- NH4 + and PO4 3−
- Co3+ and NO2 −
Answers
- (NH4)3PO4, ammonium phosphate
- Co(NO2)3, cobalt(Iii) nitrite
Nutrient and Drink App: Sodium in Your Food
The chemical element sodium, at least in its ionic course every bit Na+, is a necessary nutrient for humans to live. In fact, the homo body is approximately 0.15% sodium, with the average person having one-twentieth to one-tenth of a kilogram in their body at whatsoever given time, generally in fluids outside cells and in other actual fluids.
Sodium is too present in our diet. The mutual tabular array salt we use on our foods is an ionic sodium chemical compound. Many processed foods as well incorporate significant amounts of sodium added to them as a variety of ionic compounds. Why are sodium compounds used so much? Usually sodium compounds are cheap, but, more importantly, most ionic sodium compounds dissolve easily. This allows processed nutrient manufacturers to add sodium-containing substances to food mixtures and know that the chemical compound volition dissolve and distribute evenly throughout the food. Elementary ionic compounds such as sodium nitrite (NaNOtwo) are added to cured meats, such as bacon and cafeteria-style meats, while a compound called sodium benzoate is added to many packaged foods as a preservative. Table 3.4 "Some Sodium Compounds Added to Food" is a fractional listing of some sodium additives used in food. Some of them you may recognize after reading this chapter. Others you lot may not recognize, but they are all ionic sodium compounds with some negatively charged ion as well present.
| Sodium Chemical compound | Use in Food |
|---|---|
| Sodium acetate | preservative, acidity regulator |
| Sodium adipate | food acid |
| Sodium alginate | thickener, vegetable gum, stabilizer, gelling agent, emulsifier |
| Sodium aluminum phosphate | acidity regulator, emulsifier |
| Sodium aluminosilicate | anticaking agent |
| Sodium ascorbate | antioxidant |
| Sodium benzoate | preservative |
| Sodium bicarbonate | mineral salt |
| Sodium bisulfite | preservative, antioxidant |
| Sodium carbonate | mineral table salt |
| Sodium carboxymethylcellulose | emulsifier |
| Sodium citrates | food acid |
| Sodium dehydroacetate | preservative |
| Sodium erythorbate | antioxidant |
| Sodium erythorbin | antioxidant |
| Sodium ethyl para-hydroxybenzoate | preservative |
| Sodium ferrocyanide | anticaking agent |
| Sodium formate | preservative |
| Sodium fumarate | food acrid |
| Sodium gluconate | stabilizer |
| Sodium hydrogen acetate | preservative, acidity regulator |
| Sodium hydroxide | mineral common salt |
| Sodium lactate | nutrient acrid |
| Sodium malate | food acid |
| Sodium metabisulfite | preservative, antioxidant, bleaching agent |
| Sodium methyl para-hydroxybenzoate | preservative |
| Sodium nitrate | preservative, color fixative |
| Sodium nitrite | preservative, color fixative |
| Sodium orthophenyl phenol | preservative |
| Sodium propionate | preservative |
| Sodium propyl para-hydroxybenzoate | preservative |
| Sodium sorbate | preservative |
| Sodium stearoyl lactylate | emulsifier |
| Sodium succinates | acidity regulator, flavour enhancer |
| Sodium salts of fatty acids | emulsifier, stabilizer, anticaking agent |
| Sodium sulfite | mineral salt, preservative, antioxidant |
| Sodium sulfite | preservative, antioxidant |
| Sodium tartrate | food acrid |
| Sodium tetraborate | preservative |
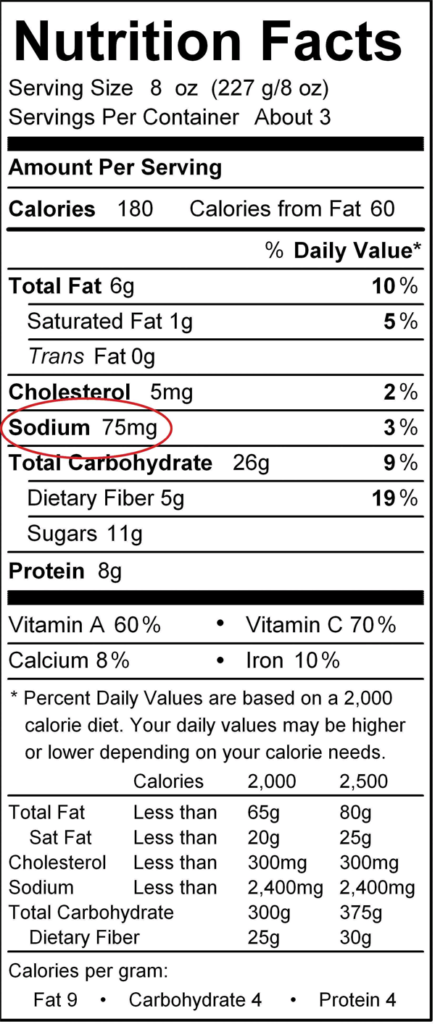
The use of so many sodium compounds in prepared and candy foods has alarmed some physicians and nutritionists. They fence that the boilerplate person consumes too much sodium from his or her nutrition. The average person needs simply nearly 500 mg of sodium every day; most people eat more than this—up to ten times equally much. Some studies accept implicated increased sodium intake with high blood pressure; newer studies advise that the link is questionable. However, there has been a push to reduce the amount of sodium most people ingest every day: avoid candy and manufactured foods, read labels on packaged foods (which include an indication of the sodium content), don't oversalt foods, and use other herbs and spices besides table salt in cooking.
- Ions course when atoms lose or proceeds electrons.
- Ionic compounds accept positive ions and negative ions.
- Ionic formulas balance the total positive and negative charges.
- Ionic compounds accept a elementary system of naming.
- Groups of atoms can have an overall charge and make ionic compounds.
Questions
- Explain how cations form.
- Explain how anions form.
- Give the accuse each atom takes when it forms an ion. If more than one charge is possible, list both.
- One thousand
- O
- Co
- Give the charge each atom takes when it forms an ion. If more than ane charge is possible, list both.
- Ca
- I
- Iron
- Give the charge each cantlet takes when information technology forms an ion. If more than one charge is possible, listing both.
- Ag
- Au
- Br
- Requite the accuse each cantlet takes when it forms an ion. If more than one charge is possible, list both.
- Southward
- Na
- H
- Proper name the ions from Exercise 3.
- Name the ions from Practice 4.
- Name the ions from Exercise five.
- Proper noun the ions from Exercise six.
- Requite the formula and proper noun for each ionic compound formed betwixt the two listed ions.
- Mg2+ and Cl−
- Iron2+ and Oii−
- Fethree+ and O2−
- Requite the formula and name for each ionic compound formed between the two listed ions.
- M+ and Southwardtwo−
- Ag+ and Br−
- Srtwo+ and N3−
- Give the formula and name for each ionic compound formed between the two listed ions.
- Cu2+ and F−
- Catwo+ and Otwo−
- Chiliad+ and P3−
- Give the formula and name for each ionic compound formed between the two listed ions.
- Na+ and Nthree−
- Co2+ and I−
- Authree+ and S2−
- Give the formula and name for each ionic compound formed between the two listed ions.
- M+ thenfour 2−
- NHfour + and Due southtwo−
- NH4 + and POfour 3−
- Requite the formula and name for each ionic compound formed betwixt the ii listed ions.
- Ca2+ and NO3 −
- Ca2+ and NO2 −
- Sc3+ and CiiH3Otwo −
- Give the formula and proper noun for each ionic compound formed between the 2 listed ions.
- Pbiv+ and so4 two−
- Na+ and I3 −
- Li+ and Cr2O7 2−
- Give the formula and proper name for each ionic compound formed between the two listed ions.
- NHiv + and Northward3−
- Mgtwo+ and CO3 2−
- Althree+ and OH−
- Give the formula and name for each ionic chemical compound formed between the two listed ions.
- Ag+ and SO3 2−
- Na+ and HCO3 −
- Fethree+ and ClOiii −
- Give the formula and name for each ionic compound formed between the ii listed ions.
- Rb+ and Oii ii−
- Au3+ and HSOiv −
- Sr2+ and NO2 −
- What is the difference betwixt Then3 and SOthree two−?
- What is the divergence betwixt NO2 and NO2 −?
Answers
- Cations form by losing electrons.
-
- 1+
- 2−
- 2+, 3+
-
- 1+
- 1+, 3+
- one−
-
- the potassium ion
- the oxide ion
- the cobalt(II) and cobalt(3) ions, respectively
-
- the silver ion
- the gilded(I) and gold(Iii) ions, respectively
- the bromide ion
-
- magnesium chloride, MgCl2
- iron(2) oxide, FeO
- iron(III) oxide, Fe2O3
-
- copper(II) fluoride, CuF2
- calcium oxide, CaO
- potassium phosphide, Yard3P
-
- potassium sulfate, GrandtwoSOfour
- ammonium sulfide, (NH4)2Southward
- ammonium phosphate, (NH4)3PO4
-
- atomic number 82(4) sulfate, Pb(SO4)2
- sodium triiodide, NaI3
- lithium dichromate, LiiiCr2O7
-
- argent sulfite, AgiiSOthree
- sodium hydrogen carbonate, NaHCO3
- iron(Iii) chlorate, Fe(ClO3)three
- And so3 is sulfur trioxide, while And thenthree 2− is the sulfite ion.
Media Attributions
Figure iii.ane
- "Kosher Salt" by stlbites.com © CC Past-ND (Attribution NoDerivs)
Source: https://opentextbc.ca/introductorychemistry/chapter/ions-and-ionic-compounds/
ارسال یک نظر for "what is the ratio of al ions to s ions in a neutral compound?"